Categories
Metrix-Secure # 66630
EVA Legless Dual Chamber Bag - Male Screw Connector, 3,000ml, 42 Per/Cs
$ 551.90 $ 551.90
Log in or create an account to proceed with checkout.
How Can We Help? Fast answers on bulk discounts, same-day shipping, and other questions for our team
Request Product Info
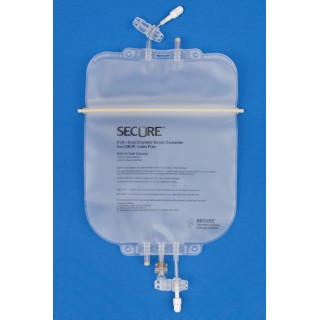
Metrix-Secure #66630 EVA Legless Dual Chamber Bag with Male Screw Connector - 3,000ml, 42 Per/Cs
The Metrix-Secure #66630 EVA Legless Dual Chamber Bag is designed for advanced parenteral nutrition and complex infusion therapies. With a total capacity of 3,000ml, this dual chamber configuration allows fat emulsions to be stored separately from other TPN components, helping support solution stability and visual inspection prior to mixing. Each case contains 42 bags to support high-acuity clinical environments and dedicated TPN programs.
Manufactured from high-quality Class VI Ethyl Vinyl Acetate (EVA) monolayer copolymer film, these bags provide strong seals, excellent flexibility, and resistance to cracking and pinholes. The legless design offers greater versatility for compounding and storage, while the male screw connector supports secure attachment to TPN compounding equipment and gravity filling systems. Each bag includes an attached non-reopening clamp to help prevent accidental opening once the line is closed.
Total Parenteral Nutrition (TPN): Dual chamber EVA containers like the #66630 are commonly used for patients who cannot receive adequate nutrition via the gastrointestinal tract due to conditions such as short bowel syndrome, obstructions, leaks, or severe malabsorption. TPN solutions are infused through central venous access devices (such as PICC lines, central lines, or implanted ports) and may be required over weeks or months, making container safety and reliability critical.
Storage recommendations: EVA dual chamber bags should be stored at temperatures below 40°C and kept away from direct sunlight, excessive heat, and humidity to preserve material performance and product integrity.
DEHP advisory: Per Infusion Therapy Standards of Practice, only non-DEHP administration sets should be used—especially when delivering lipid-based infusates (such as IVFE and TNA)—since DEHP is lipophilic and may leach into solutions, posing increased risk for neonatal, pediatric, pregnant, and long-term care patients.
Product Features:
- This product is not made with natural rubber latex or DEHP
- Ports and port tubes contain TOTM plasticized PVC
- EVA container
- Stores fat emulsion separate from other TPN components for improved inspection and stability
- Legless bags offer increased versatility
- 4000ml (500ml/3500ml)
- 3000 ml (500ml/2500ml)
- 1500 ml (250ml/1250ml)
With its dual chamber design, legless configuration, and secure male screw connection, the Metrix-Secure #66630 bag provides a high-quality, purpose-built solution for TPN and other complex IV therapies that demand both safety and flexibility.